CryoNeedle
Scar and Keloid Removal Life By Ice
Cryotherapy: Superior results from the inside out
Life By Ice
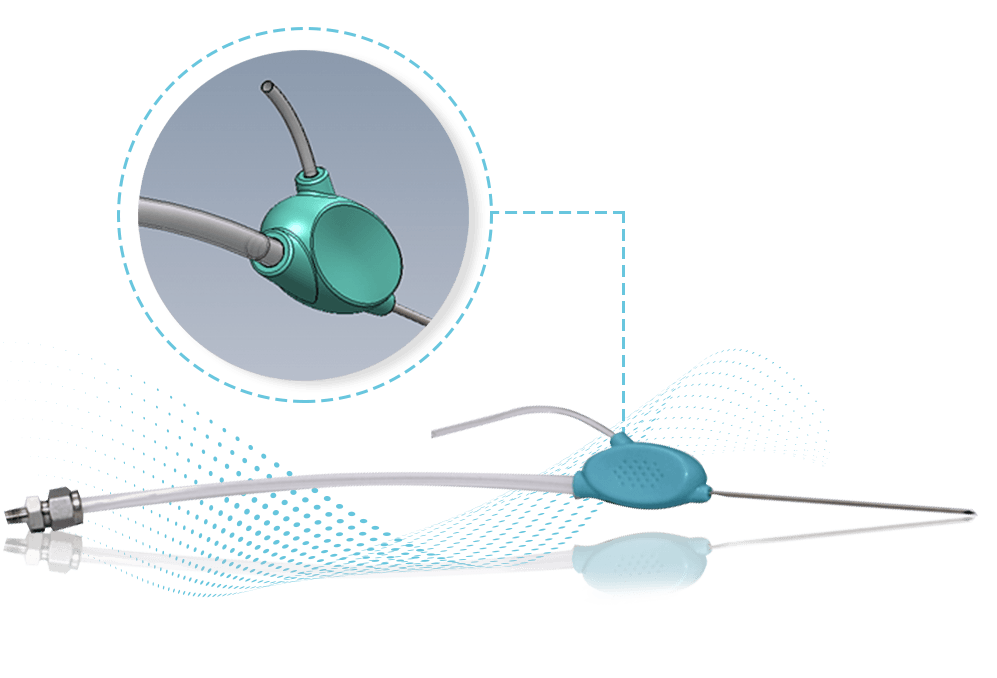
Life by Ice's CryoNeedle Cryoprobe (U.S Patent 6,503,246) is a hand-held cryosurgical instrument used to destroy the tissue of Hypertrophic Scars and Keloids (HSK). The device is used during surgical procedures by way of intralesional application of an extremely cold cryoprobe.
Cryotherapy: Innovative Concept and Advantages
The novelty of the CryoNeedle concept is both its intralesional application and its form. The device is especially elongated to enable precise application, while affording maximum user control and comfort. To enhance the intended intralesional penetration into the scar tissue, which is often hard, rubbery and dense, the CryoNeedle has a sharp pyramid-shaped, sealed, distal tip. Temperatures of -25°C to -50°C (-13°F to -58°F) can be achieved.
Clinical studies clearly demonstrated the excellent efficacy of the CryoNeedle due to the increased freezing area of deep scar material, compared with that obtained with commercially available surface/contact probes.
Cryotherapy: Moderate freezing and thawing rates improve cellular fibroblasts destruction and block the microcirculation in the tissue –two mechanisms that when applied on the deep scar material contribute to excellent results. Contact-treatments are measured in seconds since extended exposure might be harmful. However, the CryoNeedle treatment is measured in minutes due to the fact that a significantly longer exposure will not harm the skin surface, but still harm the scar internal tissue. Almost all of the HSKs treated by the contact techniques result in significant hypopigmentation, while the skin surface of the HSK treated by the intralesional CryoNeedle exhibited pronounced hypopigmentation.
Among other advantages of the intralesional application over alternative modalities is a short preparation time. In addition, the risk of infection is extremely low; wound care is minimal, while suture removal is not necessary.
For further information, visit Life by Ice.
Product Brief
Product Applications
Global Distribution
Distribution support includes includes funding for participating in exhibitions worldwide and digital marketing promotion for local distributors, as well as the exploration of new market opportunities for distribution in Brazil.
Chaban's Role
Production:
Marketing Activities:
Become a Distributor: Cryoneedle
We will get back to you as soon as possible.
Please try again later.